Leqembi for monoclonal antibodies directed against amyloid for the treatment of Alzheimer's disease
Effective for dates of service on and after July 6, 2023, Medicare will pay for Leqembi (lecanemab-irmb) for monoclonal antibodies directed against amyloid for the treatment of Alzheimer's disease.
This drug is covered under NCD 200.3 - Monoclonal antibodies directed against amyloid for the treatment of Alzheimer's disease (AD).
Medicare covers the drugs with traditional FDA approval in this class when a prescribing clinician or their staff decides the Medicare coverage criteria is met. The clinician or staff also submits information to help answer treatment questions in a qualifying study. You can participate in the CMS National Patient Registry (or another CMS-approved study) to get Medicare payment for treating your patients with Leqembi.
Part A billing instructions
Institutional claims:
- For dates of service on or after July 6, 2023, use HCPCS code Leqembi J0174 (Injection, lecanemab-irmb, 1mg).
- Type of Bill: 12X, 13X, or 85X
- Revenue Code: 0636
- Condition Code: 30
- Value Code: D4 with the national clinical trial (NCT) number "99999999" or a dedicated NCT number
- Report one of the following modifiers:
- Q0 (Investigational clinical service provided in a clinical research study that is in an approved clinical research study), or
- Q1 (Routine clinical service provided in a clinical research study that is in an approved clinical research study)
- Q0 (Investigational clinical service provided in a clinical research study that is in an approved clinical research study), or
- Diagnosis codes:
- Z00.6 (noting a registry) AND one of the following dx codes:
- G30.0 Alzheimer's disease w/early onset
- G30.1 Alzheimer's disease w/late onset
- G30.8 Other Alzheimer's disease
- G30.9 Alzheimer's disease, unspecified
- G31.84 Mild cognitive impairment
- G30.0 Alzheimer's disease w/early onset
- Z00.6 (noting a registry) AND one of the following dx codes:
Part B billing instructions
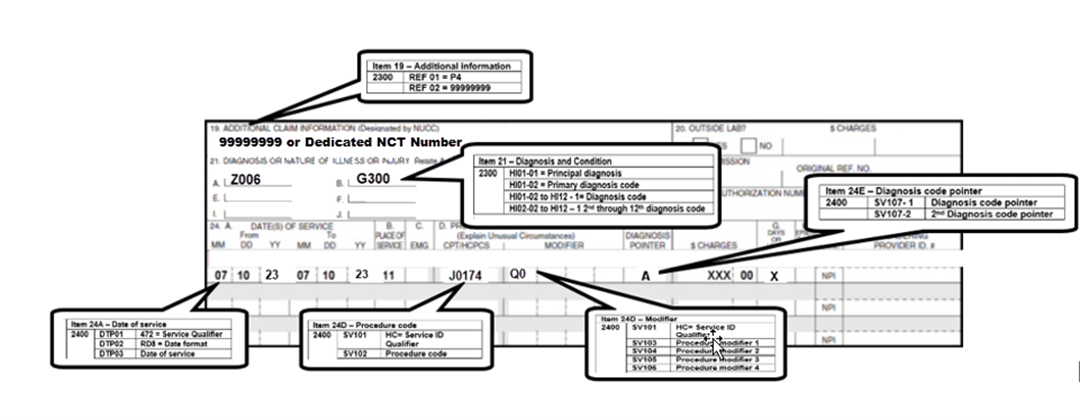
- For dates of service on or after July 6, 2023, use HCPCS code Leqembi J0174 (Injection, lecanemab-irmb, 1mg).
- Report the registry trial number (8-digit number) using "99999999" or a dedicated NCT number in the narrative description field (Item 19) or the electronic claim in Loop 2300 REF02 (REF01=P4)
- To ensure claims are submitted and processed correctly, please review the reporting information with your software vendor.
- Click here to view the CMS-1500 (02/12) data element requirements and electronic claim loop mapping.
- To ensure claims are submitted and processed correctly, please review the reporting information with your software vendor.
- Report one of the following modifiers:
-
Q0 (Investigational clinical service provided in a clinical research study that is in an approved clinical research study),
or
- Q1 (Routine clinical service provided in a clinical research study that is in an approved clinical research study)
-
- Diagnosis codes:
- Z00.6 (noting a registry) AND one of the following dx codes:
- G30.0 Alzheimer's disease w/early onset
- G30.1 Alzheimer's disease w/late onset
- G30.8 Other Alzheimer's disease
- G30.9 Alzheimer's disease, unspecified
- G31.84 Mild cognitive impairment
- G30.0 Alzheimer's disease w/early onset
- Z00.6 (noting a registry) AND one of the following dx codes:
- The diagnosis code pointer should be used to indicate the primary diagnosis on the claim form.
References