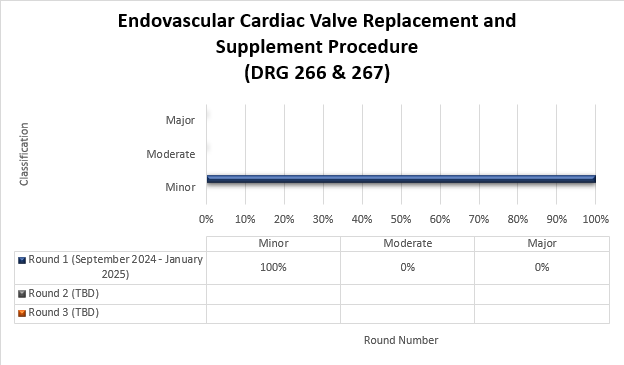
Targeted probe and educate (TPE) round results: Endovascular cardiac valve replacement and supplement procedure (DRG 266 & 267)
Top denial / partial denial reasons and high-level results are listed below from each round of DRG 226 & 267 TPE reviews that have been conducted thus far by Medical Review. If you have questions about your individual results, please contact the nurse reviewer assigned to your review for additional information. Additional rounds of review will be utilized when the targeted topic demonstrates a continued need for review with newly identified providers.
Top denial / partial denial reasons
The most common reasons for denial or partial denials are the following:
- Medical necessity – The documentation submitted does not support medical necessity as listed in coverage requirements.
- Insufficient documentation – Insufficient documentation was provided to support the services as billed to Medicare. Medical Review makes multiple attempts to correct these error types before completion of the review. Below are the following denial reasons for insufficient documentation that we were not able to resolve:
- Documentation submitted does not support the patient’s condition required the close physician supervision, the medical management to support the necessity of an IRF stay.
- Documentation does not support the preadmission screen was completed or updated within the 48 hours immediately preceding the IRF admission.
Round results